

Atomic Numberĭifferences: Atomic Number and the Atomic Mass of Elements A periodic repeating of attributes may be found in the elements with increasing mass in the same group. Similarly, at room temperature, the elements of Group 8A are unreactive gaseous. Group 1A elements, for example, are largely soft metals that are strongly reactive to water. Elements with comparable properties are assigned to the same column or group. When looking at the periodic chart, one can observe that elements are ordered by increasing atomic numbers. The number of electrons in an element significantly influences its chemical behavior. The atomic number is significant because it affects the number of electrons that surround the nucleus. Except for hydrogen, all atoms contain neutrons in their nucleus.
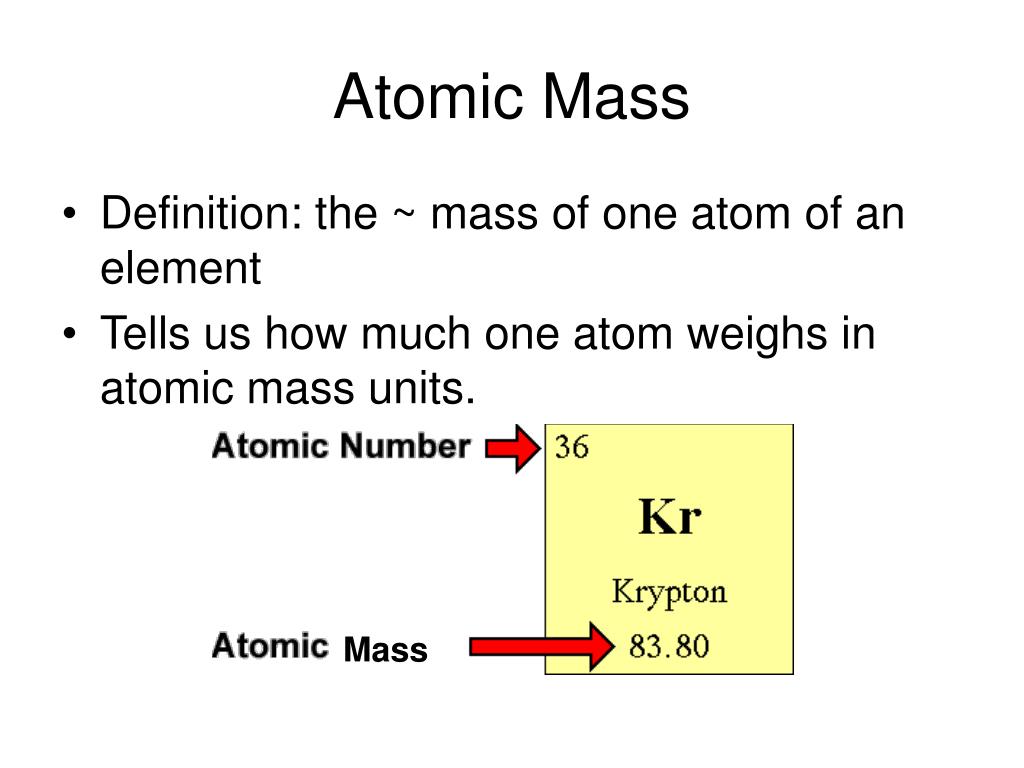
Neutrons are uncharged subatomic particles that, when bonded in an atomic nucleus, are stable. Regardless of the number of neutrons present, elements are identified based on the number of protons in the nucleus. The atomic number indicates how many protons are contained within the atom’s nucleus. The overall atomic masses presented in periodic tables, such as the one for Nitrogen, are derived for each element’s naturally occurring isotopes, weighted by the weight of those particular isotopes on Earth. This makes it a useful value to know when working with elements that have multiple isotopes. The contribution of each isotope to the normal is determined by how large a proportion of the example it makes up. Generally, the atomic mass number is rounded to the nearest whole number.īecause an element’s isotopes have different atomic weights, researchers may also determine the element’s overall atomic mass (also known as the atomic weight). The general atomic mass is the average of the atomic masses of many different isotopes. For example, a Nitrogen atom with seven neutrons and seven protons have an atomic mass of 14 AMU. What is the Atomic Mass of an Element?Ī single atom’s atomic mass is its absolute mass, and it is commonly given in atomic mass units (AMU). In this article let’s study the nuclear mass of elements in detail, along with the atomic number and atomic mass of elements. The atomic mass is the average weight of all isotopes present in an element. The molar mass of each isotope is multiplied by its abundance. It’s worth noting that it is also referred to as atomic weight. Atomic mass is the average mass of an element’s atoms measured in atomic mass units (AMU).


 0 kommentar(er)
0 kommentar(er)
